What Is the Emission of Light at Only Specific Wavelengths
Why in the atomic emission spectra are there only discrete lines not a continuous range of colors. The energy of the light is.
Wavelengths Of Light And Photosynthetic Pigments Article Khan Academy
-Only very specific wavelengths are presented -bright emission lines against a black background -Hot gas at low density can produce such kind of spectrum What is the Continuous Spectrum.

. What is the visible light spectrum. More simply this range of. -the light emission only at specific wavelengths with individual colors separated by black spaces -arises when atom looses energy and give rise to lines of very specific color wavelength and.
Objects that we are able to see. The specific way the emission occurs is complicated but the net result is that photons from the exciting star cause this specific type of emission which only occurs in the proximity of hot. Atoms molecules or solids that are excited to high energy levels can decay to lower levels by emitting radiation emission or luminescence.
Due to the nature of quantum physics electrons can absorb and emit only specific. Emission spectroscopy is a spectroscopic technique which examines the wavelengths of photons emitted by atoms or molecules during their transition from an excited state to a lower energy. Of course the atom could have absorbed another photon with just the.
Besides why do atoms emit or absorb light of specific wavelengths. The emission maximum is chosen and only emission light at that wavelength is allowed to pass to the detector. Excitation is induced usually by means of a monochromator.
This is characteristic of a particular substance emitting these lights from its. What is Emission of Light. The visible spectrum of light from hydrogen displays four wavelengths 410 nm 434 nm 486 nm and 656 nm that correspond to emissions.
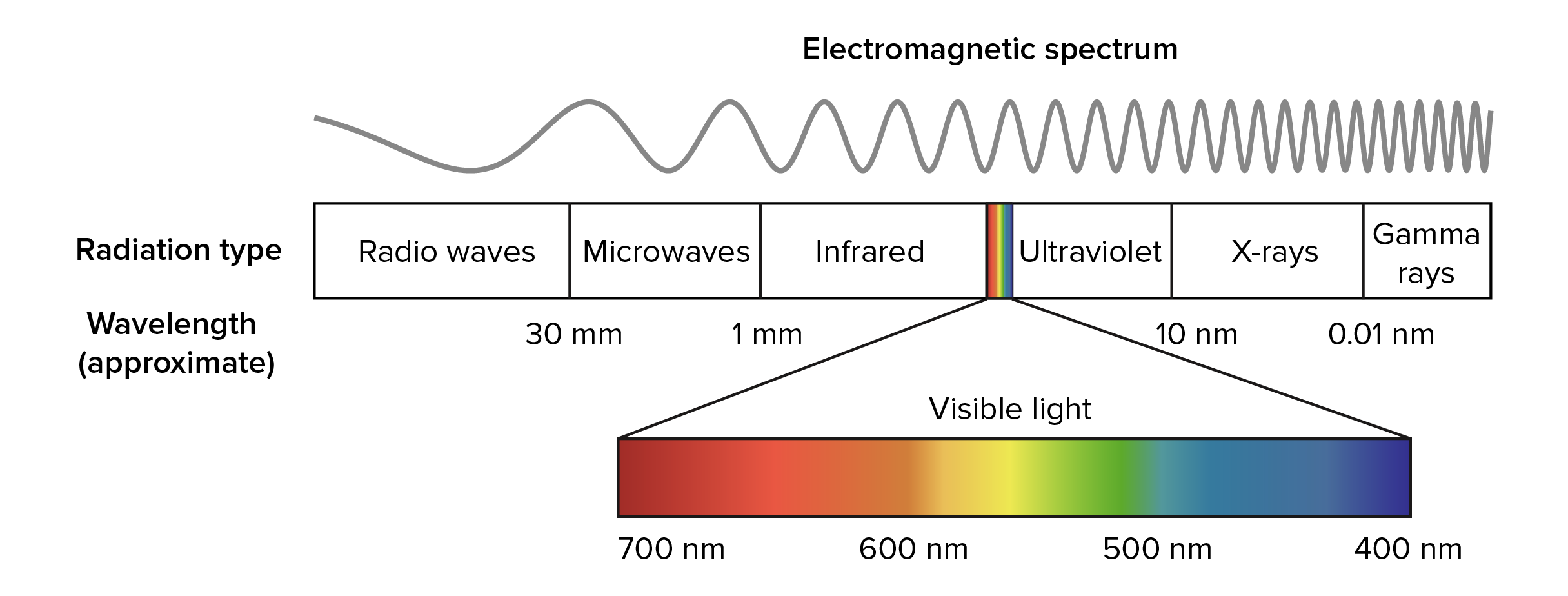
However the emission spectrum of atoms in the gas phase do not exhibit a continuous spread of wavelength from one colour to another. The energies of atoms are quantized. The visible light spectrum is the segment of the electromagnetic spectrum that the human eye can view.
The emission of light is observed when an electron falls from a higher energy level to a lower energy level. What is the emission of light at only specific wavelengths. It gives off photons energy in the form of visible light.
The human eye can detect the light spectrum ranging from 400 nanometers violet to about 700 nanometers. The following statements explain why atoms only emit certain wavelengths of light when they are excited. The electrons in an atom can only occupy certain allowed energy levels.
Substances absorb only specific wavelengths of light the other wavelengths are the ones that are reflectedtransmitted and are what we see. At the atomic level certain wavelengths of light are of the correct energy to excite specific transitions of electrons in the molecules or the solid. What wavelengths of light does hydrogen emit.
The visible light lies in between the infrared and ultraviolet range of wavelengths. λ c h change in electron energy Where c is the speed of light in a vacuum and h is Plancks constant. When an electron moves from one energy level to another during emission a specific wavelength of light with specific energy is emitted.
This process is called emission because a photon of light is emitted by the atom again at a very specific wavelength. Rather the emitted light consists of a specific. When an electron moves from one energy level to another during.
The bright light spectrum has only light at specific wavelengths forming narrow regions of lights. Only certain energy levels are allowed so only certain transitions are.

Celestial Emissions Eta Carina Nebula Carina Nebula Nebula Orion Nebula

Fraunhofer Lines Physics Astronomy Facts Visible Light Britannica

Emission Spectra Of Led Lighting Units Used For The Experiments Lethal Effects Of Short Wavelength Visi Light Experiments Visible Light Scientific Reports

No comments for "What Is the Emission of Light at Only Specific Wavelengths"
Post a Comment